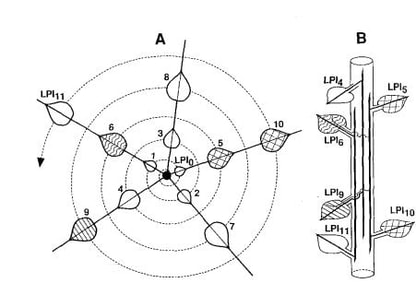
I had a special day today. The PR arm of the College of Arts and Sciences wanted to highlight me as one of a few professors in social media posts around our May 5 commencement. They also filmed me having a discussion with 12 graduating undergrads about their experience at UNCG and with me. To say the hour long conversation was profoundly fun and meaningful to me, would be a ridiculous underestimate. A few of the students had participated in undergraduate research with me- one saying in the conversation that a course in plant physiological ecology where they had to read two scientific papers each week, and the research they did my lab, took them from a somewhat lost student, to someone with tremendous focus on science, particularly conservation of marine animals. One of the other students in the conversation is working with five other students on a tobacco project. The first time I have used tobacco as a model system in 20 years. So, today I celebrate organismal biology, model systems, tobacco plants and eastern cottonwood just for the fun of it-. I am so excited to work with Tobacco again (after 20 years) with undergraduates. The tobacco plant pictured above is almost ready for the experiment! This pilot experiment is looking at whether the preference and performance of an insect herbivore feeding on tobacco plants grown in microplastic amended soils or controls. Unfortunately, I underestimated the time it would take for tobacco plants to grow, so the students are frustrated. But, they got to find a cool topic, amended soil with microplastics, designed and built plant-insect cages from PVC and netting that would have cost five times more to buy, transplanted seedlings and kept them alive. Students will finish the pilot experiment this summer. To digress for a second, I was at a seminar the other day where the opening slide showed a progression from genes, to populations to ecosystems that defined the topic. As a plant physiological ecologist, my heart sank that organisms weren't in the progression. That reminded me of the collaborative work of developmental plant anatomists with plant physiologists who took years, but brilliantly tied together the form and function of eastern cottonwood plants which allowed me to conduct a pretty fun PhD thesis and beyond. Also, tobacco is such a cool plant to work with for similar reasons. This blog post discusses some of my favorite papers using tobacco and eastern cottonwood as model systems that never got the traction they deserved in the scientific community. The work described below represents two out of five research areas my lab was focused on before becoming an administrator. Tobacco and Cottonwood My first paper, and my only single authored paper ever, was the result of a question on my PhD candidacy exam at Yale from Clive Jones, Bill Smith, John Gordon, Charles Remington and Mike Montgomery that related to my thesis using cottonwood as a model system. It was titled, Leaf development and leaf stress: increased susceptibility associated with sink-source transition. The project was based on a fully funded NSF grant I wrote with Clive. The group of friendly inquisitors asked to me look at the relationship between leaf development and susceptibility of leaves to insects and pathogens. A couple of hundred hours in the library (does anyone remember living in the stacks?), and a few hundred references, later I produced an answer that suggested that there was a window of time during leaf development associated with the sink source transition where susceptibility to specialist insects and pathogens peaked. And, that window was due to a balance of several characteristics including secondary compounds, size, toughness, nitrogen level, starch/sugar levels and was consistent across a wide range of organisms. That led to a cool paper that has recently found more interest from others. I didn't do so well on the other question they asked me, but they concluded that none of them could have answered it any better. They thought my answer to the leaf development and susceptibility to consumers was excellent, and they couldn't; argue with the funded NSF grant, and I was admitted to candidacy. One paper that I loved was led by then graduate student, and now Professor at Missouri State University (Alexander Wait;), "Chrysomela scripta, Plagiodera versicolora (Coleoptera: Chrysomelidae), and Trichoplusia ni (Lepidoptera: Noctuidae) Track Specific Leaf Developmental Stages" (https://doi.org/10.1603/0046-225X-31.5.836) where we grew tobacco (and eastern cottonwood) in sand, and were able to control the relative growth rate, of plants by providing exponentially increasing nutrients daily at the rate of the RGR we wanted. And, were able to produce plants that had leaves reaching full expansion on the stem at different leaf positions. The results supported our hypothesis that specialist insects carefully tracked leaf developmental stage (and the point of sink-source transition) independent of RGR, independent of leaf position and independent of nutrient supply. It was such a cool study. I think, unfortunately, we published it in Environmental Entomology and it hasn't been read as much as it might have. A follow up paper on how closely insects track leaf development stage, in this case tracking the feeding behavior of aphids on cottonwoods, and carefully correlating that behavior with biochemical leaf characteristics was done by former PhD Graduate Student Georgiana Gould.. She showed in the paper "Variation in Eastern Cottonwood (Populus deltoides Bartr.) phloem sap content and toughness due to leaf Development may affect feeding site Selection behavior of the aphid, Chaitophorous populicola Thomas (Homoptera: Aphididae). " The aphid seemed to track leaf development to avoid mature leaves and to preferably feed on rapidly expanding leaves. Concentrations of the amino acids GABA and aspartic acid, as well as the phenolic glycoside salicin, differ in leaves of different developmental stages and may be used by the aphid to determine the age of leaves to feed upon. Another favorite paper is, "Why it matters were ion a leaf a folivore feeds " was conducted with a wonderful undergraduate, Soren Leonard (https://www.linkedin.com/in/a-soren-leonard-4316b344/). This paper (doi.org/10.1007/BF00328818) was based on the fact that the tip of leaves stop expanding well before the base of leaves. We hypothesized that given how leaves develop and expand, herbivores feeding on the base of the leaf would appear to have eaten much more tissue than those that feed on the tip of the leaf. And that amount of area lost from a leaf would be dramatically different if herbivores fed on the base vs. tip of an expanding leaf, which could lead to reductions in plant performance. We tested this hypothesis by taking the same area of leaf tissue from the tip or base of mature and expanding tobacco leaves. We found that removing area from the base of an expanding leaf created over twice the amount of visible damage than occurred on the tip of an expanding leaf. Furthermore, damage to the base of an expanding leaf resulted in nearly a 40% reduction in the final leaf area, resulting in a 35% reduction in the number and mass of fruits produced. .Some implications of this study could be extraordinarily important in assessing the amount of leaf area eaten at a whole plant, plant population and/or ecosystem level. For example if we had tried to estimate the amount of leaf area consumed by herbivores by the size of the hole from the base of an expanding leaf, we would conclude that herbivores removed 16.6 cm 2 even though only 3.9 cm 2 was actually removed. On the other hand, if we estimated the reduction in overall leaf area simply from the size of the final hole in a leaf, we might conclude that leaf area was reduced by 16.6 cm 2 when, in fact, damage to the base of the leaf resulted in over a 180 cm 2 reduction in the area of that leaf. (over ten fold). This really could have created large errors in data assessing the amount of herbivory or loss of leaf area due to herbivores in agricultural or forest ecosystems. Although I think this is a simple paper, I also felt that its results were really important for scaling from leaf development to ecosystems. However, it is hard to measure where an herbivore eats on a leaf in the field. Despite the fact I think this paper was a really important paper (and almost immediately accepted by Oecologia- a great journal for this work), the rest of the world did not- it has a really low citation rate relative to other less interesting papers I have written with students or colleagues. And, in discussing with scientists who try to assess the amount of leaf area that herbivores remove, or the affect of herbivory on ultimate leaf area, despite the 4 fold mistake in leaf area eaten, and the 10 fold mistake in leaf area reduction, they felt these data were simply an annoyance and would just unnecessarily complicate their work and the narrative of their findings. C'est la vie. There was another paper my group published using tobacco that was also an extraordinarily cool paper. My lab was interested in the ecological and evolutionary physiology of heat shock proteins and why, given their role in thermotolerance, were low molecular weight heat shock proteins only induced by stress and not constitutively produced (that we later demonstrated protect PSII during heat stress). Because of other work that had been done, we thought it might be possible that one leaf could be heat stressed on a plant and send a volatile signal in the air or a chemical signal through the vasculature to induce the heat stress response in other leaves. Molecular heat shock protein biologists, at the time, thought we were crazy, because they believed that cells only induced HSP production when they were directly stressed. Bill Hamilton a former undergraduate and PhD student (with Sam McNaughton and me) and now professor and chair of biology at Washington and Lee tested this idea using tobacco- again because the relationships between form and function that had put together by others in the paper " Heat-shock proteins are induced in unstressed leaves of Nicotiana attenuata when distant leaves are stressed. " https://doi.org/10.2307/2657048 Much to our delight, we discovered that a systemic induction of heat-shock proteins (Hsps) occurred in response to the treatment of a leaf with heat shock, mechanical damage, or exogenous application of methyl jasmonate (MJ). All treatments increased the abundance of members of the 70-kD Hsp (Hsp70) family and induced synthesis of one or more of the small Hsps (sHsp) (16–23 kD) in both treated and untreated leaves. Those results provided the first evidence that Hsps can be systemically induced in plants and suggest that systemic induction of Hsps may be important in pre-adapting leaves to stress. Why you ask?, Although we never tested this in the field, one could hypothesize, that, for example, leaves on the eastern side of plant may experience increased temperatures before those on the western side of the plant and that systemic induction might be valuable in inducing thermotolerance before the western leaves were in direct sunlight. Again, this very simple and cool experiment that kind of shattered a paradigm at the time that cells only induce greater production of HSPs if they are directly stressed. Nonetheless, not many people cared. A short trip into the ecological and evolutionary ecology of heat shock proteins Bill, myself, and a great post doc in my lab, Scott Heckathorn, professor at the University of Toledo, found this experiment really cool, and in the early 2000s it was kind of fun to find a result inconsistent with a molecular paradigm at the time. On a side note, my lab's work with heat shock proteins was originally aimed at linking molecular and ecological approaches to understand whether their where resource costs that prevented plants from just making them all of the time (and we showed that there could be) which led to me receiving an NSF (Presidential) Young Investigator Award. A paper with Scott and Dick Hallberg (a heat shock protein biologist of note working in yeast systems) "Heat shock proteins and thermotolerance: Linking ecological and molecular perspectives," described that perspective. And, we publishes numerous papers on this topic. But, sometimes our work became the subject of ridicule because of not doing the sort of mechanistic work that molecular biologists expected. So, we were forced (and that ended up being a good thing) to get better at molecular approaches to the HSP work in order to have our ecological/evolutionary work accepted. That culminated when Scott worked with Craig Downs, Tom Sharkey and me find a result that was something I would have never predicted/imagined being done in my lab- we were the first lab to demonstrate a molecular function for how plant low molecular weight heat shock proteins protect photosystem II during heat stress (doi.org/10.1104/pp.116.1.439). Who da thunk that? Back to cottonwoods Returning from the digression, the tobacco and cottonwood studies were related to NSF and the Andrew Mellon Foundation support. And, these projects were great fun because how fun it is to work with students. It is just a bummer that what was exciting to us (or at least me) did not resonate much with other scientists. But, I am really proud of the work these students did and the potential significance of the work. One other study conducted by an undergraduate that drew on the form and function work of the special people I alluded to above but didn't name- Philip R. Larson, Jud Isebrands and Richard E Dickson- who connected the form and functional development of cottonwood probably better than any other plant species. In their work they mapped the vasculature of cottonwood trees and related the vascular connections (and flow of water of and sugar) to the phyllotaxy of leaves. Leaves in cottonwood, develop at the same angle from each other and after a few spins (depending how fast they grow) their leaves vertically align. And, that alignment is always consistent with the Fibonacci series (see figure below). Nature loves symmetry. In most of the plants we worked on the phyllotaxy was 2/5 meaning that every that after 2 spins around the stem, every fifth leaf would align, and every third leaf would be most distant from the 5th leaf. Not surprisingly the strength of vascular connections was strongest between every 5th leaf. And the fifth leave after two spins was least connected to the third leaf. In the paper "Plant vasculature controls the distribution of systemically induced defense against an herbivore," Clive Jones, Robert Hopper (undergrad), Vera Krischik and I tested whether the vascular connections between cottonwood leaves could predict the strength of an induced defensive response in other leaves when one leaf was damaged. The paper showed that mechanical damage to single leaves resulted in systemic induced resistance (SIR) in non-adjacent, orthostichous leaves (vertically aligned on the stem) with direct vascular connections, both up and down the shoot; but no SIR in adjacent, non-orthostichous leaves with less direct vascular connections. The showed the control that plant vasculature exerts over signal distribution following wounding and might be useful in predicting SIR patterns, explain variation in the distribution of SIR, and relate this ecologically important phenomenon to biochemical processes of systemic gene expression and biochemical resistance mechanisms. This paper received more citations and much more attention. But, it never would have happened with the dedication to connecting plant form and function that inspired Larson, Isebrands and Dickson. Clive, Vera an I tried to integrate and conceptually model how plant form and function could link to new models of thinking about how the interaction between plants and insects and plants and pathogens can be interpreted from a greater understanding of plant-form and function and of herbivore and pathogen characteristics in three synthetic papers, A Phytocentric Perspective of Phytochemical Induction by Herbivores. In: D. Tallamy and M. Raupp (eds.). 1991 edited volume Phytochemical Induction by Herbivores. J. Wiley and Sons. pp. 3-45; Plant Stress and Insect Herbivory: Toward an Integrated Perspective. In: H.A. Mooney, W.E. Winner and E.J. Pell (eds.) Integrated Responses of Plants to Environmental Stress. Academic Press, NY. pp. 249-282 and in one of the best papers I ever wrote but almost nobody read, Phytocentric and Exploiter Perspectives of Phytopathology. Advances in Plant Pathology 8: 149-195. ISBN: 012033710X, 9780120337101. My grand synthesis (with Clive Jones) attempted to link plant form and function, plant phenology, evolutionary characteristics of insects and pathogens to describe the evolution of insect and pathogen communities on various plants, and specific traits that would be present in specialized insects and pathogens. It was the best paper I ever wrote, Leaf Ontogeny, Plant Phenology, and Plant Growth Habit: Toward a General Theory of Resource Exploitation by Herbivores and Pathogens. But, it was rejected by the American Naturalist with one very positive review (from Sir John Lawton, my academic grandfather- you have to admit it is pretty cool to have a British Knight as a grandfather) and one negative review. After leaving it and stupidly never resubmitting because I decided to move on to new things when I started as an assistant professor, I am back to it again. I have had a few scientists in the field read that paper recently and they all strongly encouraged me to get it up to date and resubmit. I am working with undergraduates on that now. I don't know why I procrastinated so much on the work I am proudest of. Science is funny that way. So, here is to Tobacco and cottonwood- both great model systems for organismal biology. Here's to a hope that the amazing understanding we have gained at the genome level doesn't make organismal biology obsolete. And here the hope that the new undergraduates in my lab with find a joy of working with tobacco that is not related to smoking. A short synopsis of the stuff that got more attention (and not): The other three main research areas (and two peripheral areas) consuming my life and accounting for a large number of citations are: 1) understanding the role of ontogenetic drift in plant traits in understanding the function of phenotypic plasticity with Kelly McConnaughay (and several of her students) and David Ackerly- the area of science where most of my citations are. (we started with a synthesis piece, Interpreting phenotypic variation in plants) and a symposium that David and I organized with some other amazing people, produced a nice paper that still gets read quite a bit, The evolution of plant ecophysiological traits: Recent advances and future directions; (2) Another area was global change ecology (around $20million in funding)where my work is cited frequently - example Nature paper here of a large team project in the Mojave Desert.. And the third area is another area that boomeranged on me. Twenty years ago I worked with Mae Gustin (and Mae's graduate student Jody Erickson), Steve Lindbergh and Dale Johnson on a project trying to understand the flux of mercury in ecosystems, where we published a kind of seminal paper in mercury flux (using aspen-- close enough to cottonwood for comfort), "Accumulation of atmospheric mercury by forest foliage ". This came back around when I returned to the faculty at UNCG in 2021 and inherited three grants from Martin Tsui, who moved to Hong Kong, looking at mercury flux in response to different silvicultural practices used to restore loblolly pine plantations to longleaf pine ecosystems. Oh, and then there is my short stint with radishes in Hal Mooney's group- another one of my best papers that barely anyone read came from my time in Hal's lab Anthropogenic stress and natural selection: Variability in radish biomass accumulation increases with increasing SO2 dose. I also had a blast working with Sam McNaughton as as his mentee and colleague when I was an assistant professor at Syracuse, and our students including Bill and Scott, Bryan Wilsey, Michele Giovannini, Ben Tracy, Kevin Williams, Doug Frank, Greg Hartvigsen and Stephanie Moses on interactions of grasses (and other plants) with herbivores. I am particularly proud of a conceptual/synthesis paper that Scott, Sam and I wrote on C4 Plants and Herbivory. And, Greg did a really interesting paper with Alexander and I on tri-trophic interactions as a result of resource availability to cottonwood saplings. Brian wrote some really interesting papers on global change and grasses with Sam and I-- a topic for another blog about the story of my carbon dioxide work. Writing about Sam just reminded me that during the seven years I was at Syracuse University (I was tenured and promoted there). I declined every offer, every year, to go to the Serengeti with Sam and his team. That is near or on the top of the list of stupid decisions in my life. I remember the days of cottonwood and tobacco (and radish and Serengeti grasses) fondly. And, I hope future days will also make me nostalgic before I die. Some other day I'll blog about our much more appreciated work regarding allometry/phenotypic plasticity in plant traits, global change biology, and mercury biogeochemistry. Below is a diagram of a 2/5 phyllotaxy of a cottonwood sapling. The shadings relate to the experiment. People actually hand drew these figures back then-- and it wasn't me. I can barely draw a straight line.
0 Comments
Leave a Reply. |
|